by Dennis Crouch
CeramTec has now filed its petition for certiorari challenging the Federal Circuit’s affirmance of the TTAB’s cancellation of CeramTec’s “pink ceramic hip” trade‑dress registrations based upon overlapping utility patent protection. In its 2001 TrafFix decision, the Supreme Court held that a utility patent constitutes "strong evidence" that the claimed features are functional, thereby creating a presumption against trademark protection for those features. [20250820165039977_CeramTec Cert Petition]
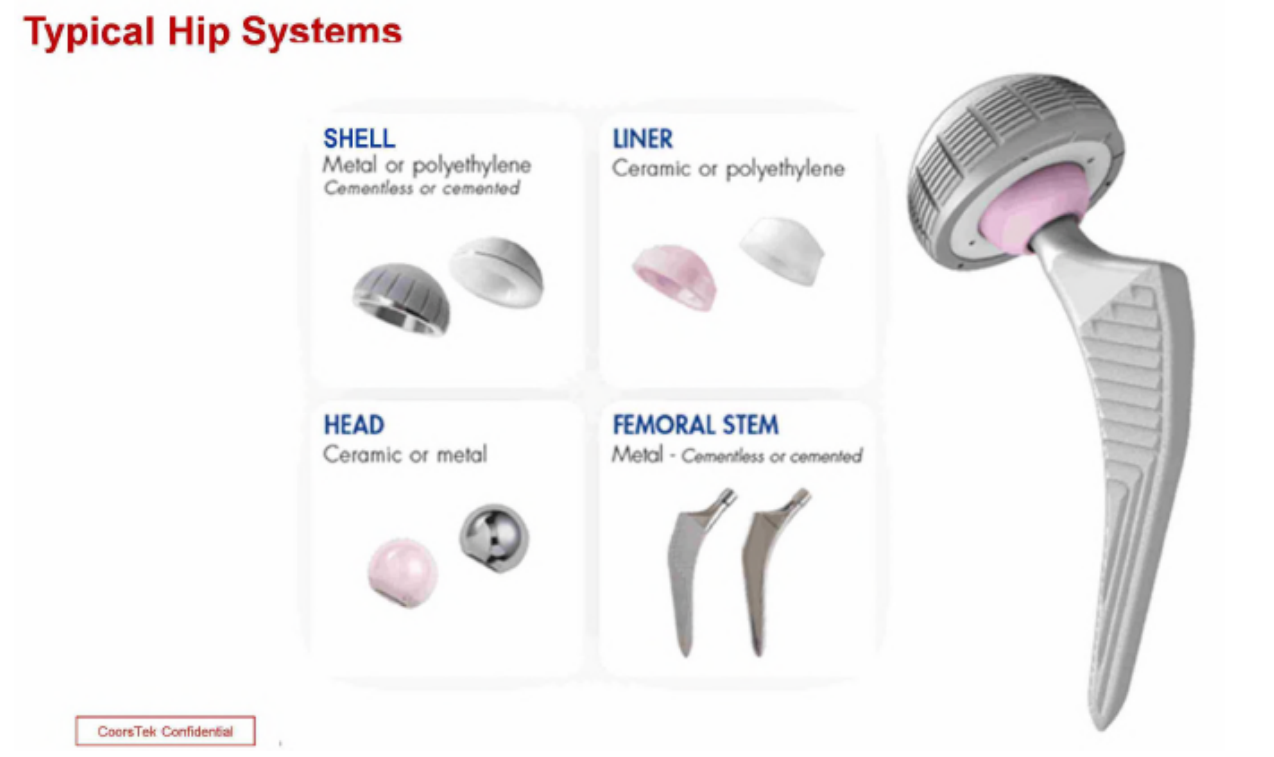
CeramTec's fake-hips are pink and the company used that in marketing is Biolox Delta product and has established market distinctiveness in the color itself. The USPTO initially agreed and registered the color pink for the ceramic bones.
The problem is that the pink color arises from from the addition of chromia to the ceramic compound -- an important aspect of the (now expired) utility patent (US5830816) covering its product. Although CeramTec admits that its pink product is covered by the patent -- and that the pink is caused by the 0.33 wt% chromia used in their product -- that result is not required by the patent. In particular, the patent does not claim or even mention the pink color and chromia addition within the claim scope can yield a spectrum of colors in zirconia‑toughened alumina (ZTA_ depending upon the concentration used, including “pink, red, purple, yellow, black, gray [or] white.” Although CeramTec chose 0.33 wt% chromia for its product, nothing in the patent indicates that concentration is unique or more beneficial than other concentrations within the claim scope.
Hips don't lie?: An additional interesting and potentially important fact. The utility patent provides a number of reasons why chromium is important as an addition to the zirconium dioxide ceramic, it:
- strengthens the alumina matrix,
- restores hardness lost by adding zirconia, and
- improves thermal and wear resistance.
However, as this lawsuit developed, CeramTec's position changed dramatically. In response to CoorsTek's cancellation petition, CeramTec argued to the Board that "although it had once believed that adding chromia provided material benefits to ZTA ceramics, that belief was mistaken and has since been disproven." This reversal was supported by experimental testing conducted by a government-sponsored research agency in related German litigation, which found that adding chromia at various levels (0.0, 0.1, 0.3, and 0.5% by weight) had no effect on hardness or wear resistance.
This alteration in utility means that chromia - although claimed - is not actually functional. This is highlighted by the CoorsTek competing product which achieves the same ZTA features without chromia. CoorsTek though wanted to ensure that the ortho purchasers understand its product is effectively the same as CeramTec's and so added pink dye in order to guarantee visual similarity.
In 2014 CoorsTek petitioned to cancel CeramTec’s color registrations as functional. After some delay because of parallel litigation, the TTAB ultimately granted cancellation, and the Federal Circuit affirmed. The TTAB applied the Morton‑Norwich framework and also credited TrafFix’s “strong evidence” rule. It concluded that the patent record (and other materials) showed chromia conveyed utilitarian advantages to ZTA and that pink is a “natural byproduct” of the concentration CeramTec chose. On that view, the expired patent became “strong evidence” that the pink trade dress is functional. The Board also found CoorsTek's criticisms of the testing methodology and scope to be persuasive.
On appeal, the Federal Circuit held that TrafFix does not demand that the patent explicitly claim the exact design feature (pink) or that the marked feature be the “central advance” of the patent; it sufficed that “the addition of chromia causes ZTA ceramics to become pink.” The court treated the “central advance” phrasing in TrafFix as illustrative rather than as a threshold requirement. I summarized the Federal Circuit’s decision when it issued, noting the cautionary lesson for patent prosecutors: statements in a utility patent about functional benefits can later loom large in a trademark functionality fight.
- Dennis Crouch, "Cert Petition Preview: Federal Circuit's Broad Reading of TrafFix in CeramTec," Patently-O (June 19, 2025)
- Dennis Crouch, "Federal Circuit: Pink Hip Implants Are Functional, Cannot Be Protected as Trade Dress," Patently-O (Jan. 8, 2025)
- Dennis Crouch, "Pink Ceramic Hip Implants: When Functionality Trumps Trade Dress," Patently-O (Oct. 10, 2024)
The new petition for writ of cert to the Supreme Court asking:
Question Presented: Whether under this Court’s decision in TrafFix Devices, Inc. v. Marketing Displays, Inc., 532 U.S. 23 (2001), a utility patent that produces a product with a wide array of designs is “strong evidence” that every aspect of every design produced by practicing the patent is functional rather than arbitrary, incidental, or ornamental.