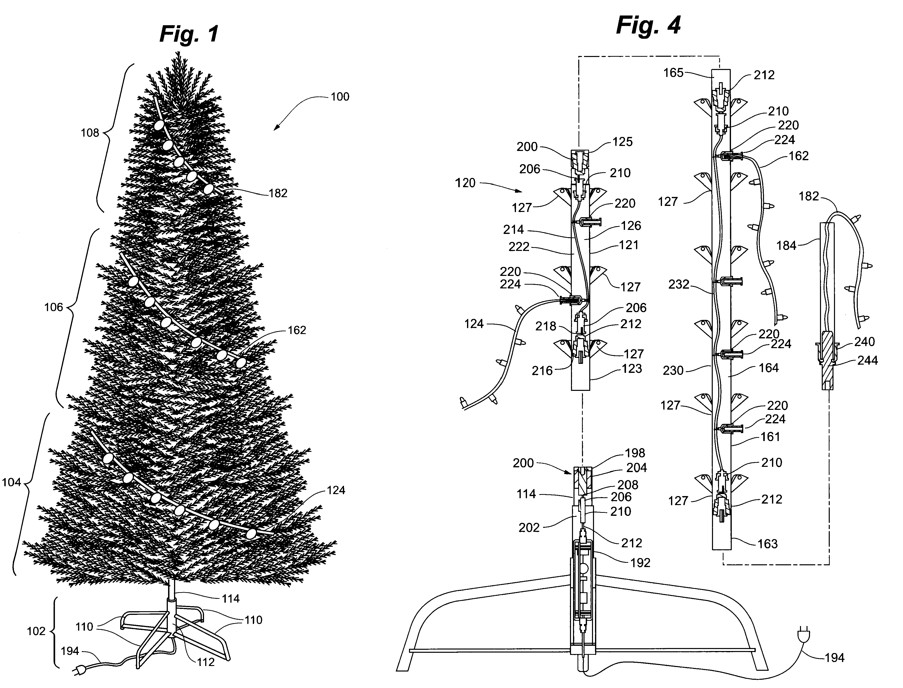
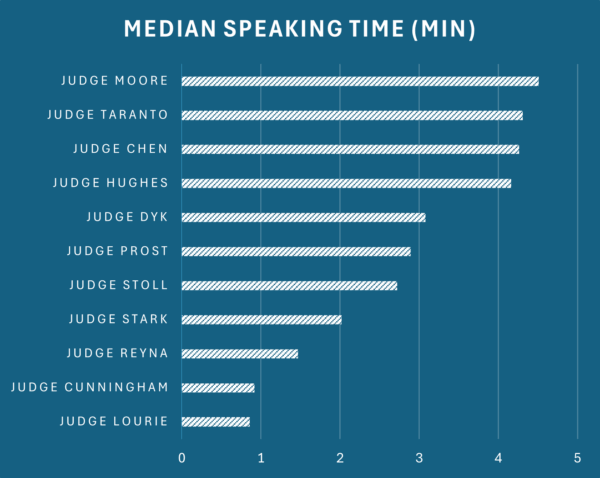
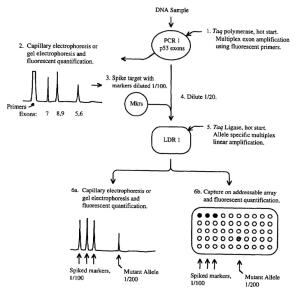
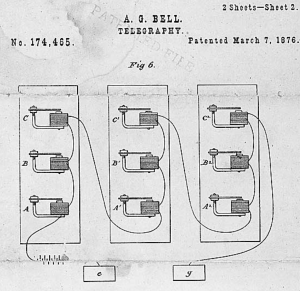
by Dennis Crouch
For many years, I relied on certpool.com to keep tabs on the Supreme Court’s certiorari docket. When that site went dark, I started building my own tracker in 2024, running it locally on my personal computer and checking it daily for new and interesting cases before the US Supreme Court. That project has now grown into SCOTUSGate.com, which I’m making publicly available for the first time. The site is very much in alpha, a work in progress, but it is functional and I’d welcome feedback from readers as it develops.
SCOTUSGate tracks petitions for certiorari and shadow-docket activity across the full Supreme Court docket. The database (named Cerberus, after the three-headed dog who guards the gates of the underworld) currently shows over 1,000 pending petitions, with structured data on conference scheduling, cert-stage briefing, CVSG invitations, and case dispositions. The site pulls docket information directly from the Court several times per day and I have incorporated both traditional text analysis tools as well as AI to extract questions presented from petition PDFs and assign topic tags for filtering and discovery.
The name works on a few levels. A gate as portal: the site is a point of entry into information about what the Court is considering. A gate as barrier: the certiorari process is itself a gate, with the Court granting review in only a small fraction of cases. And in the Watergate tradition of institutional-accountability naming: a site built on the conviction that the Court’s work should be visible and open to scrutiny, particularly at the cert and shadow-docket stages where transparency is most limited. (more…)