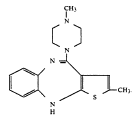
In a much anticipated decision, Judge Richard Young of the Southern District of Indiana has ruled that Eli Lilly’s patent covering the drug Zyprexa is valid, enforceable and infringed. The patent is not scheduled to expire until 2011.
The well-written 224 page opinion reads like a biography of Patent No. 5,229,382, the Zyprexa patent. In particular, the court details the prosecution history of the patent and provides a full analysis of the prior art references that the generic manufacturers cited against the patent. In the end, court determined that the generics could not prove invalidity or unenforceability:
Defendants have failed to prove by clear and convincing evidence that claims 1, 2, 3, 7, 8, and 15 of the ’382 patent are invalid as anticipated under 35 U.S.C. § 102, as obvious under 35 U.S.C. § 103, under the double patenting doctrine, or as barred by prior public use under 35 U.S.C. § 102. Defendants have further failed to prove by clear and convincing evidence that the ’382 patent is unenforceable for inequitable conduct.
The defendant generic manufacturers had stipulated that if the ’382 patent is valid and enforceable, then their actions constitute infringement. Thus, the court found that their ANDA submissions constituted an act of infringement.