Tag Archives: Federal Circuit En Banc
Appeals Court Affirms Unenforceability of Lemelson’s Patents Due to Prosecution Laches
RIM, with the support of Canada and Intel, Ask Court for Another Review of BlackBerry Patent Case
Supreme Court: FTC confronts decision that liberally allows brand-generic patent settlements
Patently-O Tidbits
Claims definite unless “insolubly ambiguous.”
BlackBerry Case Makes Major Precedential Changes
CAFC initiates mediation program
Patent Drafting In the Wake of Phillips v. AWH — Initial Thoughts
Claim Construction — Dictionaries are Still OK.
En Banc Federal Circuit Panel Changes The Law of Claim Construction
BlackBerry Patent Lawsuit Rises Again; NTP Responds to Patent Office Rejections
On Remand: Knorr-Bremse District Court Finds Willfulness Even Without Adverse Inference Rule
SmithKline Beecham v. Apotex: A Conclusion?
Pain for Purdue Pharma: CAFC affirms inequitable conduct finding
Government Opposes Supreme Court Review in Festo Remix
PATENT REFORM: Preventing Inequitable Results Under Knorr-Bremse Willfulness
This post is part of our continuing series discussing a US House of Representatives working draft of patent legislation, by Marcus Thymian, Jennifer Swartz, and Dennis Crouch.
In the landmark decision of Knorr-Bremse v. Dana Corp., an en banc panel of Federal Circuit judges recently held that no adverse inference is to be drawn from the absence of an opinion of counsel in willfulness determinations. This ruling overruled the Court’s earlier precedent in Quantum and was a dramatic change. The proposed legislation attempts to balance this swing in jurisprudence by adding a section on willfulness that incorporates the Knorr-Bremse holding while carving out broad exceptions.
The proposed changes repeat the Knorr-Bremse holding that “[t]he absence of an opinion of counsel shall not create an inference that the infringement was willful.” Further, the legislation makes mere knowledge of a patent or its contents insufficient for a finding of willfulness against the defendant.
The legislation diverges from Knorr-Bremse by listing “other factors” that may be considered in increasing damages against an infringer. Included as one such factor is intentional copying by a defendant who has specific knowledge he is infringing patented subject matter. However, unlike Knorr-Bremse, the defendant must rebut proof of specific knowledge by proffering evidence of an “informed good faith belief” that the patent is invalid, not infringed, or unenforceable (i.e. an opinion). Thus, while no adverse inference of willfulness will be drawn in the absence of an opinion under the proposed changes, the defendant will still have the onus to provide an opinion in cases in which the plaintiff proves the defendant’s specific knowledge of infringement.
Finally, the legislation also proposes adding to the statute for increased damages the “factor” of prior litigation between the parties for substantially similar infringement. Thus, in a later action between parties for colorably similar infringing conduct, willfulness may be based on the knowledge the infringer has maintained since the earlier litigation.< ?xml:namespace prefix ="" o />
Antitrust: FTC confronts decision that liberally allows patent settlements
In a March 8 decision, the 11th Circuit Court of Appeals set aside an FTC order that barred Schering-Plough from settling an infringement suit with generic makers over the patented blood pressure drug K-Dur. The FTC had concluded that the settlement was an “unreasonable restraint of trade.” The 11th Circuit disagreed, finding that because the suit involved patented products, neither a per se nor a rule of reason analysis would be appropriate.
By their nature, patents create an environment of exclusion, and consequently, cripple competition. The anticompetitive effect is already present. “What is required here is an analysis of the extent to which antitrust liability might undermine the encouragement of innovation and disclosure, or the extent to which the patent laws prevent antitrust liability for such exclusionary effects.” Therefore, in line with Valley Drug, we think the proper analysis of antitrust liability requires an examination of: (1) the scope of the exclusionary potential of the patent; (2) the extent to which the agreements exceed that scope; and (3) the resulting anticompetitive effects.
Applying their rule to the facts, the Court concluded that payment from a patent holder to a generic competitor cannot be the sole basis of a violation of antitrust law. Accordingly, the court SET ASIDE the decision of the Federal Trade Commission and VACATED its cease and desist order.
Petition en banc: Now, the FTC has filed a petition for rehearing of the case en banc. In the petition, the Commission raises three primary contentions:
- Not every patent infringement litigation settlement should be shielded from antitrust scrutiny, so long as entry of the allegedly infringing product is not precluded at any time subsequent to expiration of the patent;
- That the decision subverts the goals of Hatch-Waxman; and
- That the decision used an improper standard review of critical disputed facts.
If this petition for rehearing fails, the Government is expected to appeal to the Supreme Court.
Links:
- Download the FTC petition
- Download Schering Antitrust Decision.pdf
- Patent at issue, No. 4,863,743
- NYTimes Article
- PubPat Amicus Brief to the 11th Circuit [pdf]
Full Panel Denies Rehearing in Fosamax Patent Case
Merck & Co. v. Teva Pharmaceuticals (Fed. Cir. 2005) (on petition for rehearing).
Merck & Co. owns the patent on the best-selling drug Fosamax. Teva, a generic manufacturer lost an infringement case at the district court after the court found the patent valid and infringed. In January 2005, the Court of Appeals for the Federal Circuit reversed the district court — vacating the lower court’s finding of validity and infringement.
Merck, with the amicus support of PhRMA, subsequently filed a petition for rehearing en banc. The CAFC has denied that the request, thus maintaining the panel decision.
DISSENT: In a two-page dissent, Judge Lourie, supported by Chief Judge Michel and Judge Newman, argued that the panel decision erroneously concluded that commercial success was not probative to obviousness in this case and by linking commercial success with the failure of others.
Commercial success is a fact question, and, once it is established, as found here by the trial court, the only other question is whether the success is attributable to the claimed invention (“nexus”), rather than to other factors such as market power, advertising, demand for all products of a given type, a rising economy that “lifts all boats,” etc. It is not negatived by any inability of others to test various formulations because of the existence of another patent. Success is success. The panel’s rule is especially unsound in the context of an improvement patent, as here, because it holds in effect that commercial success for an improvement is irrelevant when a prior patent dominates the basic invention.
Commercial success is also independent of any “failure of others,” as that is another, separate secondary consideration.
Respectfully, the full court should have reheard the appeal to eliminate the confusion in the law that the panel opinion creates.
Interestingly, Judge Rader dissented in the three-judge panel opinion, but now agrees with the majority that the case should not be reheard.
The case will be remanded to the district court on April 28, 2005.
A REUTERS article indicates that Merck & Co. plans to petition the Supreme Court to hear an appeal from the CAFC decision.
Links:
SmithKline Beecham v. Apotex part VI
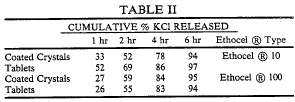
Rather than offering any opinion, the CAFC en banc decision simply vacated the court’s decision. At the same time that the en banc order was released, the three-member appellate panel released their rewritten opinion that avoided the experimental use issue entirely by finding an alternative grounds for finding the patent invalid. Specifically, the court (J. Rader) found that the patent was “inherently anticipated.”
What makes the case even more odd is that, Judge Newman’s dissent from the order declining to hear the case en banc was essentially a written as a dissent from the new Rader decision. Judge Newman criticized the new opinion’s expansion of the doctrine of inherent anticipation, arguing that the “breadth of the panel’s theory of inherent anticipation contravenes long-established precedent.” According to the dissent, if a compound’s existence “is not reasonably known to persons of skill in the field, its later discovery cannot be retrospectively ‘inherently anticipated.’”
According to the new decision, however, inherent anticipation does not require a person of ordinary skill in the art to recognize the inherent disclosure in the prior art at the time the prior art is created. Thus, it appears that a defendant, in order to invalidate a patent, must simply show that, more likely than not, a natural result flowing from an operation taught in the prior art would result in the claimed product.
In this case, the recreated panel found that the prior art was sufficient because it disclosed the manufacture of PHC anhydrate that naturally results in the production of the claimed compound (PHC hemihydrate). However, the court stated that a mere possibility of inherency is not sufficient for anticipation. (Citing Schering Corp.).
In concurrence, Judge Gajarsa found that the manufacture of PHC hemihydrate could be “a natural physical process” occurring “under normal climactic conditions and with no human intervention,” and thus found that it was an unpatentable process of nature.
In short, patent claims drawn broadly enough to encompass products that spread, appear, and “reproduce” through natural processes cover subject matter unpatentable under Section 101–and are therefore invalid.
Gajarsa’s concurrence is a foreshadow to the next few years where patentable subject matter will become a controversial area once again.
Links:
- File Attachment: SKB Apotex en banc order (49 KB)
- File Attachment: SKB Apotex Panel Decision (230 KB)
- SKB Apotex Original Panel Decision (From Georgetown Law Center)
- File Attachment: SKB Apotex District Court.pdf (2443 KB)
- Discussion of the original panel opinion: Part I, Part II, Part III, and part IV.
- Discussion of the en banc order and new appellate opinion: Part V, Part VI
- Howard Bashman provides an excellent commentary on the case via one of his readers.
- Information on SKB’s pay-out in the class action antitrust suit. (Link).