by Dennis Crouch
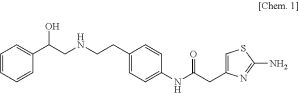
The Federal Circuit's recent decision in Astellas v. Sandoz emphasized the importance of the "party presentation principle" -- that, for the most part, courts should rely upon the parties to frame the issues that need a ruling. Visiting Delaware, Nebraska District Court Judge Bataillon had issued a sua sponte ruling that certain pharmaceutical patent claims were invalid under 35 U.S.C. § 101 -- even though the defendant had not moved for such a ruling. On appeal, the Federal Circuit has vacated and remanded -- "trusting" that on remand Judge Bataillon "will take an objective, measured, and thorough look into the legal issues and evidence of record to resolve only those disputes that exist between the parties."
Judge Bataillon's colorful opinion language asserted that the branded and generic pharma companies have colluded to pervert the intent of Hatch-Waxman. On remand, it may be substantially drier.
To continue reading, become a Patently-O member. Already a member? Simply log in to access the full post.