Tag Archives: Subject Matter Eligibility
Hindsight Bias
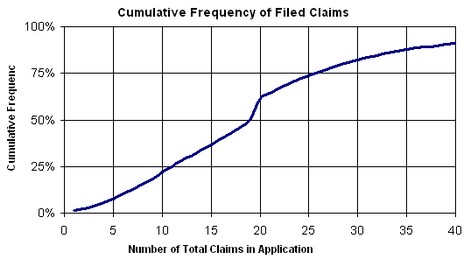
Evidence Based Prosecution I: Sensitivity to Claim Fee Variation
Scholarship: Patent Thickets See a New Light
Supreme Court: LabCorp Briefing Round I [UPDATED]
First-to-Invent vs The Constitution [Updated]
USPTO Releases New Business Method Guidelines: Requires “physical transformation” or “concrete and tangible result”
Patent Board Eliminates “Technological Arts” Requirement For Business Method Patents
Ninth Circuit Upholds Semiconductor Chip Protection Act Claim Asserting Protection for Logic Groupings
Metabolite Labs versus LabCorp — Request for Certiorari.
The Metabolite Labs case is procedurally interesting because the Supreme Court appears to be focused in a question that is quite different from the one raised by Metabolite in its petition for certiorari. From a practical standpoint, this case has the potential to once again shatter our current notion of patentable subject matter.
In February, the Court invited the Solicitor General to file a brief expressing the views of the United States on the question of whether a medical diagnosis method patent is invalid as non-patentable subject matter under Diamond v. Diehr, 450 U.S. 175, 185 (1981) (holding that one cannot patent laws of nature, natural phenomena, and abstract ideas). The patent at issue claims a method of detecting a vitamin deficiency in a human body by using a device to determine whether there is an elevated amino acid level.
The court expressed its interest in the patentability question at a point that was too late for amicus to file briefs on the issue, however, some parties have been lobbying the office of the Solicitor General to submit a brief in their favor. The Public Patent Foundation, for instance, has issued a statement outlining its lobbying efforts asking the government to support the proposition that the method is unpatentable and invalid. Of course, to my knowledge, PubPat has never been publicly in favor of the validity of any patent.
In the opinion below, the CAFC (Rader, J.) affirmed jury verdict of indirect infringement and breach of contract, and affirmed the district court’s award of over $8 million in damages to Metabolite (including damages for willfulness). The dissent (Schall, J.) argued that claim interpretation had been mishandled. Metabolite Laboratories, Inc. v. Laboratory Corp. of America Holdings, dba as LabCorp., 370 F.3d 1354 (Fed. Cir. 2004) (read my summary). The question that the High Court is now considering does not appear to have even been addressed by the CAFC decision. The Court’s docket shows that the case has already been distributed for conference five times since January 1, 2005.
The SG is expected to file a brief in the case. However, even with SG support, the court is still unlikely to take this one up.
For general information, Professor Eileen Kane’s article on the patentability of Genes and DNA provides an excellent analysis of issues surrounding patentable subject matter. Kane argues that the genetic code should be characterized as a law of nature. Available at SSRN, 71 Tennessee Law Review 707.