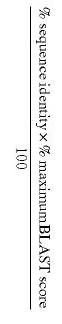
By Donald Zuhn
In an appeal from a final rejection, the PTO Board reversed rejections based on both the written description and enablement requirements of 35 U.S.C. § 112, first paragraph, and entered a new ground of rejection under 35 U.S.C. § 112, second paragraph one of the claims. (U.S. Application No. 09/915,694).
Pointedly, the Board found that claims directed to a naturally occurring amino acid (or polynucleotide) sequence at least 95% identical to the disclosed amino acid (or polynucleotide) sequence were enabled and met the written description requirement.
To continue reading, become a Patently-O member. Already a member? Simply log in to access the full post.