by Dennis Crouch
Adnexus Inc. v. Meta Platforms, Inc., No. 2024-1551 (Fed. Cir. Dec. 2025).
The district court dismissed Adnexus infringement complaint for failure to state a claim. On appeal here, the Federal Circuit has vacated and remanded, holding the district court erred by implicitly construing a disputed claim term against the patentee without first providing an opportunity to be heard on claim construction. Although pleading standards have shifted against patentees in recent years, this case is an important reminder that even legal questions require procedural fairness. Although claim construction has been (almost entirely) a question of law for the court since Markman, that designation does not bypass ordinary due process requirements. Parties are still entitled to notice and an opportunity to be heard before a court adopts a construction that dooms their case.
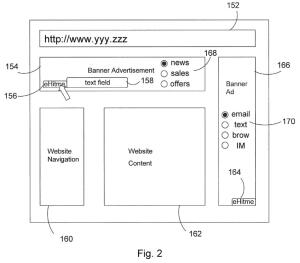
Adnexus sued Meta in the Western District of Texas, alleging that Meta's "Lead Ads" product infringes U.S. Patent No. 8,719,101, which claims a system for delivering targeted online advertisements. The key dispute on appeal centered on claim limitation [1f], which requires retrieving a "user profile" containing "delivery method preferences and demographic information." Adnexus alleged that when a Facebook user clicks on a Lead Ad, Meta's system checks cookies on the user's device and retrieves profile information, including the user's email address and other contact information, to populate prefilled forms. Meta moved to dismiss, arguing that "contact information" is distinct from "delivery method preferences" and therefore Adnexus failed to plausibly allege infringement of this limitation. The district court agreed, finding "contact information sufficiently distinct from delivery method preferences" to render the allegations implausible, and dismissed with prejudice.
On appeal, Judge Stark held that the district court's determination was an implicit claim construction that should not have been made without giving the parties notice and an opportunity to address the proper meaning of the disputed terms.
To continue reading, become a Patently-O member. Already a member? Simply log in to access the full post.