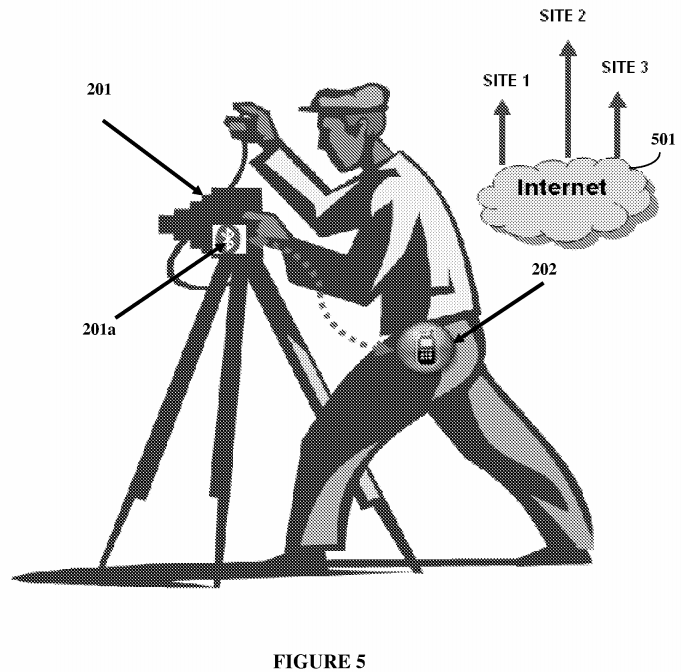
Garmin USA, Inc., et al. v. Cellspin Soft, Inc. (Supreme Court 2019)
Question Presented: Whether patent eligibility is a question of law for the court that can be resolved on a motion to dismiss, notwithstanding allegations in a complaint that the asserted claims are inventive.
[Petition for Writ of Certiorari][Appendix].