Government Opposes Supreme Court Review in Festo Remix
Overlapping Ranges Create Prima Facie Case of Obviousness
CAFC Interprets Knorr-Bremse — Finds Willful Infringement
In one of the first willfulness decisions since Knorr-Bremse, the Court of Appeals for the Federal Circuit (CAFC) has affirmed a district court’s denial of JMOL after a jury verdict of willfulness.
This case involves a mechanical money sorter that differentiates according to coin diameter. Imonex sued several defendants and won a jury verdict that included increased damages for willfulness.
According to the CAFC, “willfulness requires a showing that the totality of the circumstances evince the egregious conduct that constitutes willful infringement.” (citing Knorr-Bremse).
In this case, Imonex had disclosed the patented device (that was properly marked) to employees of the defendants several years before filing suits. Likewise, Imonex brochures and literature included statements that the products were patented. These facts, according to the CAFC, were sufficient to raise a duty of due care.
“The jury could have reasonably concluded that several instance recounted in the record triggered the [Defendants’] duty of care.”
Once the duty of care exists, the defendants must take steps to ensure that they do not violate the patent.
Affirmed.
Link:
BPAI interprets closed transition phrase for method claims
Ex parte Hideaki Ueda (BPAI ) (NOT PRECEDENTIAL)
In a case involving a process for preparing an organic electroluminescent element, the patent examiner issued a rejection for indefiniteness under 35 USC 112, second paragraph. The examiner indicated that the claimed language of "consisting essentially of" excluded any further steps which materially affect the claimed process. Because the dependent claims included further limitations, the examiner rejected those claims as logically excluded by the "consisting" language.
On appeal at the BPAI, the Board reversed the examiner, finding that the phrase "consisting essentially of" does not limit a claim only to steps specifically recited in the claim — but is only would exclude ingredients which would materially affect basic and novel characteristics.
In this case, the limitations described in the dependent claims were disclosed in the specification. As such, those elements cannot contradict the basic characteristics of the invention.
Link:
Caterpillar Petitions Supreme Court on Issue of Implied Bias of Jury Members
Caterpillar v. Sturman (on petition for writ of certiorari).
In 2004, the Court of Appeals for the Federal Circuit vacated a jury verdict in a trade secret and contract dispute involving Caterpillar. [Article]. The problem involved Juror No. 3, whose spouse was a current Caterpillar employee — and thus potentially biased.
Normally, the question of whether an individual juror is biased is a factual determination entitled to deference on appeal. However, the CAFC ruled that the district court’s decision was based on “implied bias, as opposed to actual bias” and thus is reviewed de novo. Applying Seventh Circuit law, the court found the implied bias is demonstrated by “showing that the juror has a personal connection to the parties or circumstances.” As such, the spouse’s presence on the jury created an implied bias sufficient to vacate the judgment.
Now, Caterpillar has asked the U.S. Supreme Court to review the case. In its petition for writ of certiorari, Caterpillar argues that the CAFC’s decision to “automatically disqualify the spouse of an employee of a party” is in conflict with the Second, Fourth, Fifth, Sixth, Eighth, Seventh and Eleventh circuits. The petition cites Smith v. Phillips (1982) for the proposition that “implied bias doctrine is not viable for parties challenging jurors on a per se basis. Caterpillar also argues that Sturman should not be able to argue implied bias because the company failed to use its peremptory challenges to protect against potentially biased jurors.
In its brief filed in opposition, Sturman argues that (i) there is “no circuit split” on the issues raised by Caterpillar; (ii) that the CAFC properly applied Seventh Circuit and Supreme Court law; and (iii) that Caterpillar did not raise its current argument below. Sturman notes that the implied bias has a long history of application “stretching back to Blackstone’s day.” Sturman also dismisses the importance of the case — since the CAFC’s interpretation of Seventh Circuit law is not binding precedent.
Patent: Allegation of Copying Precluded Without Evidence That Product Embodies Ideas or Design of an Asserted Claim
In Leapfrog Enters. v. Fisher-Price, Inc., (D. Del. 2005), a magistrate judge granted defendant Fisher-Price’s motion in limine to preclude plaintiff, Leapfrog, from alleging and introducing evidence of copying by Fisher-Price. In particular, the magistrate judge refused to allow Leapfrog to introduce at trial the fact that Fisher-Price possessed Leapfrog’s LeapPad product during the development of the accused product.
One factor of willful infringement is defendant’s deliberate copying of plaintiff’s ideas or designs, including copying of the commercial embodiment of the claims (and not just the claims themselves). In Leapfrog, the magistrate found that, as a threshold matter, Fisher-Price’s possession of the LeapPad product during development of the accused product was relevant to the issue of willful infringement. The magistrate still granted Fisher-Price’s motion to exclude such evidence, however, because Leapfrog had no expert testimony to prove that the LeapPad product embodied four out of the five claim elements of the asserted claim. Thus, because Leapfrog could not “close the evidentiary loop” and connect its product to the claims, it was irrelevant whether Fisher-Price copied Leapfrog’s product.
The Leapfrog Court held that although it was not necessary for Leapfrog to show that its LeapPad product was an embodiment of “every” limitation of the asserted claim, a plaintiff must adduce “some” evidence amounting to more than “mere attorney argument” that its product is an embodiment of the “ideas or design” of an asserted claim.
Links:
CAFC: Court Can Only Correct Prima Facie Patent Errors
Group One owns patents on a device for curling ribbon. Hallmark manufactures pre-curled ribbon products and in 1997, was sued by Group One for infringement.
A jury found the patent valid and infringed and awarded $8.9 million in damages to Group One. However, in a post-verdict ruling, the Judge found the patents invalid.
I. Correctable Mistake on the Patent
One of Group One’s patents included a printing error by the PTO that resulted in the omission of important claim language that was added during prosecution of the application. The district court held that it lacked authority to correct the error and that only the PTO could correct the printing error. 35 U.S.C. 254.
The appellate court affirmed, finding that a district court may only correct a patent document if the error is “apparent from the face of the patent.”
II. Motivation to Combine
The patent claims require a “stripping means” that was found in several prior art references. None of the references, however, related to ribbon curling. The jury found that the references did not obviate the claims — a verdict that was overturned by the district court in a JMOL. The CAFC restored the jury verdict, finding that even the examiner’s failure to consider stripping means references could not “provide a basis for overturning the jury’s factfinding.”
Res Judicata Must be Determined Separately for Each Asserted Patent
After a long litigation proceeding involving multiple dismissals and re-filings, a Florida district court granted summary judgment to the defendants on the grounds of Res Judicata for each of Abbey’s three asserted fuel-injection patents. One of the patents, although related to the others, had issued after Abbey’s claims had been initially dismissed.
On appeal, the CAFC found that the continuation patent could not be the subject of a dismissal for res judicata.
Because each patent asserted raises an independent and distinct cause of action, and because on this record, Mr. Abbey’s claims of infringement of the ’977 patent were never considered by the district court, they are not part of the final judgment that concluded the first lawsuit. Accordingly, a claim of infringement based on the ’977 patent is not precluded by that judgment and can be the subject of a second lawsuit.
Vacated and remanded.
Claim Term of “Conventional Computer” Limited to Computers Available as of Patent’s Effective Filing Date
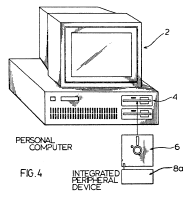
PC Connector sued SmartDisk for infringement of its patent directed to the connection of peripherals to a computer via a coupler inserted into the disk drive. The claims include limitations such as “an input/output port normally connectable to a conventional computer input/output port.”
SmartDisk argued that its flash memory cards could not infringe the patent because, like other terms in the claims, the terms “normally” and “conventional” must be interpreted as referring to technology that existed at the time of the invention. The district court agreed, finding that there could be no literal infringement because flash memories were not in normally connectable at the time the patent was filed in 1988.
On appeal, the Court of Appeals for the Federal Circuit affirmed, finding that a claim’s “meaning must be interpreted as of its effective filing date.” As such, the appellate panel held that SmartDisk did not infringe because “even a casual observer, [the flash memories] cannot be connected to a vintage 1988 computer I/O port that uses a multi-pin connector, there can be no literal infringement.”
Prosecution Tip: Do not use the modifiers such as “normally,” “traditional,” or “conventional” in your claims. Such limitations do not allow you to overcome any prior art but do severely limit the scope of the claims.
Patent Marking: Statute Requires Good Faith Belief that Mark is Properly Applied
Clontech sued Invitrogen for falsely marking its biologic products. Under 35 U.S.C. Section 292, falsely marking a product with a patent number or even the word “patent” or “patent pending” is an offense if done with the intent of (i) counterfeiting the mark of a patentee or (ii) deceiving the public. The fine is capped at $500 per offense. At the district court, Invitrogen was found liable for false marking.
On appeal, the CAFC panel noted that this case raises “virtually an issue of first impression.” After reviewing the evidence presented at trial, the panel reversed-in-part, finding clear error in the trial court’s finding that certain test results had put Invitrogen on notice of the falsity of its marks relating to reverse transcriptase.
Regarding its cDNA library products, Invitrogen argued that Section 292 “does not require a good faith belief that the marked article falls within the subject matter” of the claims. The CAFC disagreed with that argument, finding that from a policy perspective, marking should be reliable to permit free and fair competition and commerce. Thus, the appellate panel affirmed the district court ruling that the cDNA library products were falsely marked.
Blade Wars: Gillette Wins Latest Round in Multi-Blade Razor Patent Litigation
Gillette’s patent disclosing a three-blade razor also covers a four-blade version.
Gillette Co. v. Energizer Holdings Co. (Fed. Cir. 2005).
By Baltazar Gomez
Gillette owns U.S. Patent No. 6,212,777 for wet-shave safety razors with multiple blades. Gillette sued Energizer in the United States District Court for the District of Massachusetts alleging Energizer’s QUATTRO®, a four-bladed wet-shave safety razor, infringed claims of the patent. The district court denied Gillette’s motion for a preliminary injunction because it found that the claims covered only a three-bladed razor. On appeal, CAFC vacated the district court’s decision and remanded for further proceeding.
The ’777 patent claims a disposable safety razor with a group of blades, each blade placed in a particular geometric position relative to the other blades. Claim 1 describes a progressive blade exposure as follows:
A safety razor blade unit comprising a guard, a cap, and a group of first, second, and third blades with parallel sharpened edges located between the guard and cap… (emphasis added).
In determining the meaning and the scope of claim 1, the CAFC attempted to place the claim language in its proper technological and temporal context. The Court reasoned that the inventors’ statutorily-required written description in the patent itself, including the claims, the specification and the prosecution history, is the primary source of the meaning of disputed claim language.
Using this standard, CAFC determined that the language “comprising . . . a group of first, second, and third blades” can encompass the four-bladed Energizer razors. To begin, CAFC noted that the claim uses “open” claim terms “comprising” and “group of” in addition to other language to encompass subject matter beyond a razor with only three blades. Moreover, although the specification focused on blade units with three blades, the patent also disclosed a plurality of blades showing that the ‘777 patent covers razors with more than three blades. The CAFC further explained that it may be that a four-bladed razor may be less preferred embodiment, but noted that a patentee typically claims broadly enough to cover less preferred embodiments as well as more preferred embodiments, precisely to block competitors from marketing less than optimal versions of the claimed invention.
Further, CAFC also noted that the specification provided further support for interpreting claim 1 to encompass razors with more than three blades. The first sentence of the written description teaches that the invention relates to razors having a plurality of blades. Moreover, the prosecution of patents related to the ’777 patent also supports reading claim 1 as an open claim. Gillette endorsed an open interpretation of “comprising” when it argued to the European Patent Office (EPO) that a virtually identical claim in Gillette’s European counterpart to the ’777 patent would not exclude an arrangement with four or more blades. Accordingly, the CAFC concluded that the district court erred in limiting the claims of the ’777 patent to encompass razors with three blades because no statement in the ‘777 patent excludes a four-bladed razor.
In dissent, Judge Archer argued that claim 1 should not be construed as permitting a group with more than three blades simply because claim 1 contains the open transition term “comprising” in its preamble. Judge Archer concluded that a three-bladed razor is not merely a preferred embodiment of the invention, but is the invention itself, and that the inventors did not regard a blade unit with four blades arranged in the described geometry as their invention.
Note: Dr. Baltazar Gomez is a scientific advisor at MBHB in Chicago. Dr. Gomez obtained his PhD in biochemistry from the University of Texas and researched retrovirology as a PostDoc at Cornell University.
Case Questions Fundamental Questions of Patentability Requirements of Nucleic Acid Molecules
In re Fisher: EST Patentability Redux
By Donald Zuhn, Ph.D. (Patently-O Guest Author).
On Tuesday, May 3, 2005, the Federal Circuit will hear In re Fisher, in which the Court will address the utility requirement for the first time since the Patent Office set forth revised Utility Examination Guidelines in January 2001. Specifically, in Fisher, the issue of patentable utility is being raised with respect to nucleic acid molecules. In commenting on the possible importance of this case, Harold Wegner has described Fisher as having "the potential of being either the single most important pharmaceutical patent case in recent years – or a yawn." Amicus briefs filed by recognized biotech and pharmaceutical companies such as Affymetrix, Eli Lilly, and Genentech in support of the Board’s decision in Ex parte Fisher suggest that it may be the former as opposed to the latter.
The particular controversy presented in Fisher can be traced back as far as 1991, when a group of NIH investigators led by J. Craig Venter sought to protect thousands of DNA sequences corresponding to portions of expressed genes. Venter called these gene fragments expressed sequence tags, or ESTs. Venter’s group sought to protect not only the ESTs themselves, but also the full-length sequences from which the ESTs were derived and the protein products encoded by the full-length sequences, without first determining the biological function of the encoded protein products. In several applications filed on its ESTs, the NIH asserted a number of utilities, including the design of oligonucleotides for use in chromosomal analysis, PCR amplification, and recovering the corresponding full-length gene. After receiving a second rejection on its initial filing, the NIH abruptly abandoned its attempts to protect the ESTs, and withdrew all of its pending EST applications from consideration.
While the withdrawal of these applications temporarily quieted the debate surrounding EST patentability, the Patent Office again stoked the fires of controversy in 1995, when it published new Utility Examination Guidelines. The new Guidelines removed some of the obstacles to EST patenting by only requiring that an applicant assert a utility that was "specific" and "credible." The new Guidelines had thus omitted the requirement that the assertion of utility also be "substantial," as set forth by the Supreme Court in 1966 in Brenner v. Manson. In 1997, the Patent Office further declared that since ESTs were acknowledged to have utility apart from the full-length sequences from which they were derived, an applicant would no longer be prevented from securing protection for an EST by the failure to specify the function of the full-length sequence from which that EST was derived.
The Patent Office reversed course again in 2001 when it published revised Utility Examination Guidelines, reinstating the Brenner substantial utility prong. The revised Guidelines now required that an applicant assert a specific and substantial utility for the claimed invention that would be considered credible by a person of ordinary skill in the art. The Patent Office also issued Revised Interim Utility Guidelines Training Materials, which provided Examples indicating how the revised Guidelines were to be applied to thirteen different types of biochemical subject matter, including ESTs, as well as definitions of the three utility prongs. In particular, the Training Materials defined "specific utility" as utility that is specific to the subject matter claimed, as contrasted with a general utility that would be applicable to the broad class of the invention; "substantial utility" as utility having a "real world" use; and "credible utility" as utility that is believable to a person of ordinary skill in the art based on the totality of evidence and reasoning provided.
In the appeal to be heard Tuesday, appellants Dane Fisher and Raghunath Lalgudi (employees of Monsanto Co., the real party in interest) seek to reverse the Board’s decision affirming the final rejection of a claim directed to five ESTs isolated from maize leaf tissue. The five ESTs constitute only a small portion of the 4,013 sequences that appellants originally claimed and an even smaller portion of the 32,236 sequences that appellants disclosed in their application. Appellants also asserted a number of utilities for the claimed ESTs in their application, including the use of the ESTs to identify polymorphisms (i.e., alternate forms, or alleles, of the claimed sequences), to design oligonucleotide probes or primers for use in isolating DNA sequences from other plants and organisms, and to measure mRNA expression levels in plant cells using microarray technology.
The Board, in Ex parte Fisher, analyzed Brenner and subsequent CCPA and CAFC decisions in In re Kirk, In re Ziegler, In re Jolles, Cross v. Iizuka, and In re Brana, and determined that "[r]ather than setting a de minimis standard, Section 101 requires a utility that is ‘substantial’," or in the words of the Brenner court, "one that provides a specific benefit in currently available form." The Board then examined appellants’ asserted utilities and determined that none of the claimed ESTs provided a specific benefit in its currently available form. In particular, with regard to appellants assertion that the claimed ESTs could be used to measure mRNA expression levels in plant cells using microarray technology, the Board declared that "the asserted utility of the claimed nucleic acid – as one component of an assay for monitoring gene expression – does not satisfy the utility requirement of Section 101."
In briefing the issues before the Federal Circuit, appellants argue that the Board erred in concluding that an EST is "subject to a heightened standard of utility . . . that hinges upon some undefined ‘spectrum’ of knowledge about the function of the gene that corresponds to the EST." Appellants also contend that the Board erred in concluding that the claimed ESTs lack patentable utility "despite the undisputed existence of eight scientifically useful applications for the claimed ESTs and a commercially successful industry built upon the sale and licensing of ESTs corresponding to genes of unknown function, just like those at issue here." In arguing against the Patent Office’s application of a "heightened standard" of utility in this case, appellants note that the Patent Office has set forth "three substantially different constructions of the utility standard over the last decade alone," and that the Board has adopted a test "so ambiguous and impracticable that even the PTO cannot articulate with any reasonable certainty when the claimed ESTs – or any other EST – might be entitled to patent protection." Appellants also contend that the Patent Office has applied the wrong test in determining whether there is an assertion of specific utility, since "the specific utility prong only requires the existence of an identifiable benefit for the claimed invention; it does not require a benefit that is unique to the claimed invention."
The Patent Office, on the other hand, denies that appellants’ ESTs have been subjected to a heightened standard, arguing instead that appellants merely failed to assert a specific and substantial utility for the claimed ESTs that would be considered credible by a person of ordinary skill in the art. In its brief, the Patent Office often focuses on appellants’ failure to satisfy the specific utility prong, arguing, for example, that appellants’ asserted utilities "would apply not only to the over 32,000 ESTs Fisher discloses, and to the over 600,000 ESTs disclosed in Monsanto’s [six] related appeals, but also to any ESTs derived from any organism." In particular, the Patent Office counters appellants’ argument that because each EST only specifically binds to its complement, the specific sequence of each EST makes its use as a probe or primer specific, by stating that "there is no specific reason for using the EST to bind its complement," and therefore, "[t]o the extent that more sequence data could be acquired by using the ESTs as probes, that result would likely be true for any scrap of DNA derived from nature." Finally, in responding to appellants’ assertion that ESTs have a real world value as part of a multi-billion dollar industry, the Patent Office contends that "batches of ESTs of unknown significance are sold for the purpose of finding targets worthy of further development, not because the individual ESTs have any specific currently available benefit."
NOTE: This post was written by patent attorney Donald Zuhn, PhD. Don is a true expert in cutting edge biotech patent law and practices both prosecution and litigation at MBHB LLP in Chicago. (zuhn@mbhb.com). His article "DNA Patentability: Shutting the Door to the Utility Requirement," published in the summer 2001 issue of the John Marshall Law Review, contains a more thorough discussion of the history of the utility requirement, particularly with respect to the patentability of DNA sequences.
Hynix Fails to Beat Rambus on Collateral Estoppel Issue
Hynix Semiconductor v. Rambus (N.D. Cal. 2005).
In an earlier lawsuit against Infineon, a court dismissed Rambus’s patent claims in a bench statement that "I have concluded that [Infineon] has proved, by clear and convincing evidence, a spoilation that warrants dismissal of [the patent infringement case] as the only appropriate sanction after having considered the alternatives." However, the case settled before the judge issued a written opinion.
Now, Hynix argues that Rambus should be collaterally estopped from re-asserting the spoilage issue. The N.D. Cal. disagreed with Hynix, finding that the elements for a finding of collateral estoppel were not met.
In the 9th Circuit, a party asserting collateral estoppel must show four elements:
- Full and fair opportunity to litigate in the previous action;
- Issue was actually litigated in that action;
- Issue was lost in a final judgment; and
- The person against whom collateral estoppel is asserted in the present action is in privity with the party from the previous action.
Using these elements, the court found (i) that the issue of spoilage (unclean hands) is particular to each litigation and thus had not been litigated in the Infineon case; and (ii) that the Infineon court’s statements from the bench did not constitute a "final judgment" because they were not "sufficiently firm."
While there is no question that Judge Payne was sufficiently settled in his decision that he was willing to dismiss Rambus’s patent claims at the conclusion of the evidentiary hearing on spoliation, he never issued a reasoned opinion, either written or oral, explaining his ruling. Before Judge Payne could issue such a ruling, Infineon and Rambus settled the action between them, stipulating to a dismissal with prejudice.
Seemingly to protect against the CAFC’s veto power, the opinion noted that even if all the elements of collateral estoppel were met, the court would use its discretion to deny the motion because such offensive uses of collateral estoppel are disfavored.
Hynix’s motion to dismiss Rambus’s patent claims for unclean hands on the basis of collateral estoppel is denied.
Antitrust: FTC confronts decision that liberally allows patent settlements
In a March 8 decision, the 11th Circuit Court of Appeals set aside an FTC order that barred Schering-Plough from settling an infringement suit with generic makers over the patented blood pressure drug K-Dur. The FTC had concluded that the settlement was an “unreasonable restraint of trade.” The 11th Circuit disagreed, finding that because the suit involved patented products, neither a per se nor a rule of reason analysis would be appropriate.
By their nature, patents create an environment of exclusion, and consequently, cripple competition. The anticompetitive effect is already present. “What is required here is an analysis of the extent to which antitrust liability might undermine the encouragement of innovation and disclosure, or the extent to which the patent laws prevent antitrust liability for such exclusionary effects.” Therefore, in line with Valley Drug, we think the proper analysis of antitrust liability requires an examination of: (1) the scope of the exclusionary potential of the patent; (2) the extent to which the agreements exceed that scope; and (3) the resulting anticompetitive effects.
Applying their rule to the facts, the Court concluded that payment from a patent holder to a generic competitor cannot be the sole basis of a violation of antitrust law. Accordingly, the court SET ASIDE the decision of the Federal Trade Commission and VACATED its cease and desist order.
Petition en banc: Now, the FTC has filed a petition for rehearing of the case en banc. In the petition, the Commission raises three primary contentions:
- Not every patent infringement litigation settlement should be shielded from antitrust scrutiny, so long as entry of the allegedly infringing product is not precluded at any time subsequent to expiration of the patent;
- That the decision subverts the goals of Hatch-Waxman; and
- That the decision used an improper standard review of critical disputed facts.
If this petition for rehearing fails, the Government is expected to appeal to the Supreme Court.
Links:
- Download the FTC petition
- Download Schering Antitrust Decision.pdf
- Patent at issue, No. 4,863,743
- NYTimes Article
- PubPat Amicus Brief to the 11th Circuit [pdf]
Full Panel Denies Rehearing in Fosamax Patent Case
Merck & Co. v. Teva Pharmaceuticals (Fed. Cir. 2005) (on petition for rehearing).
Merck & Co. owns the patent on the best-selling drug Fosamax. Teva, a generic manufacturer lost an infringement case at the district court after the court found the patent valid and infringed. In January 2005, the Court of Appeals for the Federal Circuit reversed the district court — vacating the lower court’s finding of validity and infringement.
Merck, with the amicus support of PhRMA, subsequently filed a petition for rehearing en banc. The CAFC has denied that the request, thus maintaining the panel decision.
DISSENT: In a two-page dissent, Judge Lourie, supported by Chief Judge Michel and Judge Newman, argued that the panel decision erroneously concluded that commercial success was not probative to obviousness in this case and by linking commercial success with the failure of others.
Commercial success is a fact question, and, once it is established, as found here by the trial court, the only other question is whether the success is attributable to the claimed invention (“nexus”), rather than to other factors such as market power, advertising, demand for all products of a given type, a rising economy that “lifts all boats,” etc. It is not negatived by any inability of others to test various formulations because of the existence of another patent. Success is success. The panel’s rule is especially unsound in the context of an improvement patent, as here, because it holds in effect that commercial success for an improvement is irrelevant when a prior patent dominates the basic invention.
Commercial success is also independent of any “failure of others,” as that is another, separate secondary consideration.
Respectfully, the full court should have reheard the appeal to eliminate the confusion in the law that the panel opinion creates.
Interestingly, Judge Rader dissented in the three-judge panel opinion, but now agrees with the majority that the case should not be reheard.
The case will be remanded to the district court on April 28, 2005.
A REUTERS article indicates that Merck & Co. plans to petition the Supreme Court to hear an appeal from the CAFC decision.
Links:
Microsoft ordered not to release patented TCP software
In an April 12 decision, Judge Jeffrey White of the Northern District of California granted Plaintiff Alacritech’s motion for a preliminary injunction against Microsoft — thus preventing Microsoft from releasing its TCP Chimney or Longhorn software. Alacritech’s patent covers a software method of establishing a TCP connection with reduced processor load and accelerating digital communication.
In the thirteen page decision, Judge White determined that the patent was likely to be found valid and infringed and that the balance of hardships weighed in favor of the plaintiff, Alacritech. Factors considered include:
- A finding of likelihood of success on the merits leads to a presumption of irreparable harm in patent cases;
- Because Alacritech is a small start-up, it would not be able to withstand infringing competition from Microsoft; and
- Microsoft has not yet released the TCP Chimney software
Links:
Patent Applicant’s Claim Takings Claim Barred by Res Judicata
Back in 1986, Mr. Alton Hornback applied for a patent on a error slope sensor for a missile. The invention was found to be patentable but was withheld from issuance because of a secrecy order under 35 USC 181. The secrecy order was renewed annually until 1999, and the patent finally issued in June of 2000.
Hornback requested compensation for the delay in issuance, arguing that it was a taking. Because Hornback had argued the takings issue in a previous case, the CAFC found that his claim was now barred by res judicata.
Hornback argued that the present case raised new issues because the military had de-classified then reclassified the technology in the interim. The CAFC rejected that argument, however, finding that the classification action internal to the military did not alter his rights — “because only the Commissioner of Patents may rescind a secrecy order.”
In addition, the Appellate Panel agreed that the Court of Federal Claims “does not have subject matter jurisdiction of an adverse ruling of the PTO director.”
28 U.S.C. §1361 vests “original jurisdiction” for the issuance of mandamus orders in the district courts, authorizing the district court to hear any action “to compel an officer or employee of the United States or any agency thereof to perform a duty owed to the plaintiff.”
Dismissal affirmed.
Aside: In 1996, Hornback made another precedent when the CFC held that a pro se patent applicant without legal training is not held to the same standard as trained counsel.
Link:
Merck v. Integra: Supreme Court Hears Drug Development Patent Case
On April 20, 2005, the Supreme Court heard oral arguments in the case of Merck KGaA v. Integra (Statutory safe harbor for drug development activity). However, the oral arguments do not appear to have raised any new issues that will impact the case.
The High Court was critical of the Federal Circuit prose:
- “not a crystal clear opinion by any means” (J. Ginsburg).
- “pretty foggy” (J. Breyer).
These remarks did not extend to the substance of the opinion. Book-makers expect that the CAFC’s decision will be at least partially reversed — the real question is the eventual breadth of the statute.
In January 2005, Tom Mauro of the Legal Times reported that Justices Sandra Day O’Connor and Stephen Breyer did not participate in the decision to grant cert. Interestingly, both Justices took part in the oral arguments.
A decision is expected this summer.
Links:
-
Patently-O: Information on the case
Written Description: Defendant’s Own Expert Deposition Testimony Used to Overturn Summary Judgment of Invalidity
By John Smith
Loral, the owner of U.S. patent 4,537,375 (the ‘375 patent), sued Lockheed for infringement of claim 1. The District Court ruled in favor of Lockheed, finding the claim invalid for violating the written description requirement of 35 U.S.C. § 112.
The ‘375 patent is directed to an improved method for maintaining the orientation and attitude of a satellite in space by a process known as station-keeping. After an initial thrust, the satellite re-checks its position, and often fires its thrusters again to better correct its position. Station-keeping thus uses the limited fuel supply on board a satellite and contributes to shortening the life of satellites. The ‘375 patent discloses a method directed to improving the efficiency of the corrective procedure.
Claim 1 of the ‘375 patent entails a multi-step procedure of thruster-firing, data storage, checking of position and correction of position, and minimization of error in correction of position. Essentially, the satellite fires its thrusters to correct its position, then checks its position and compares its current error in position to its previous error in position before firing its thrusters again. Lockheed argued that the patent was invalid, because the second step of claim 1 was not adequately described in the specification.
Defendant Lockheed’s expert, when asked at deposition where one would find the second step description, answered (over counsel objections) that the second step was depicted in Item 96 of Figure 2B. The court found this, along with the testimony of plaintiffs’ expert, established that the specification sufficiently described the claim.
Lockheed also argued that the second step of claim 1 was not inherent in the written description because the specification did not state that the second step was necessarily used in the satellite position correction procedure. On appeal, the Federal Circuit noted that the second step of the claim at issue in the procedure comes only (if at all) after thrusters are fired and actual position error and historical position error are compared. According to the Federal Circuit, this “does not diminish the descriptive content of the specification.”
The Federal Circuit reversed the finding of invalidity, and remanded back to the U.S. District Court for the Northern District of California for further proceedings.
John Smith is an attorney at MBHB LLP in Chicago. He earned both his JD and PhD (inorganic chemistry) from Vanderbilt University. He has co-authored numerous articles and served as a faculty member in the Chemistry Department of Lipscomb University in Nashville, Tennessee.
Notes:
-
In his Case Alert, David Long of the Howrey firm correctly noted that this case should be “kept in mind by litigators taking or defending technical expert depositions on written description issues.”