by Dennis Crouch
In Contour IP v. GoPro, the Federal Circuit has reversed a Judge Orrick (N.D.Cal.) summary judgment of ineligibility. The case here should be one for patent drafters to consider -- particularly thinking about how to incorporate specific technological improvements into their patent claims and specification (while still maintaining broad claim coverage). Of course, the patentee here has the benefit of actual hardware beyond mere processing.
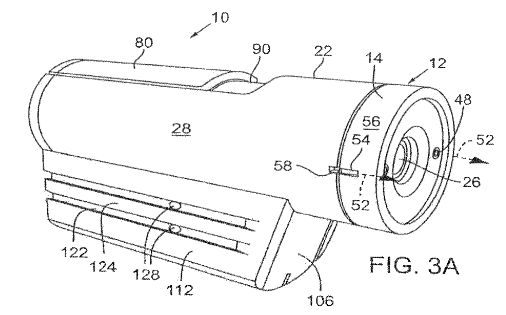
Contour owns two patents related to POV video cameras. These patents claim a camera system having lenses, sensors, etc., that generates two video streams of different quality in parallel, wirelessly transmitting the lower-quality stream to a remote device for real-time viewing and control on your phone. The higher-quality stream is stored on the camera for later use.
To continue reading, become a Patently-O member. Already a member? Simply log in to access the full post.