SmithKline Beecham Corp. v. Apotex Corp. (Fed. Cir. 2005) (en banc order)
Paxil(R) is a blockbuster drug sold by SmithKline Beecham (SKB). SKB’s patent covering the drug was initially invalidated in an opinion by 7th Circuit Judge Richard A. Posner who sat by designation as a district court judge. On appeal, a Federal Circuit panel (J. Rader) rejected almost all of Judge Posner’s reasoning, but affirmed the invalidation decision on other grounds.
Now, the CAFC issued an en banc decision to vacate the panel’s original decision with regard to the issue of experimental use, and the panel (J. Rader) has issued its new decision.
This case involves involved PHC anhydrates (without water) and PHC hemihydrates (some water). In the 70’s PHC anhydrates were invented in the UK. In 1984, SKB invented PHC hemihydrates and eventually patented a PHC hemihydrate compound. (U.S. Patent No. 4,721,723). In 1998, Apotex filed an ANDA to market a prior art PHC anhydrate. The question of experimental use arises because SKB performed clinical trials that occurred more than one year prior to filing of the patent application. Normally, a public use of a patented invention more than a year before filing invalidates the patent under 35 U.S.C. §102(b). However SKB has argued that the clinical trials were a form of experimental use.
In its new decision, the CAFC majority simply avoided the issue of experimental public use in totality. Rather, they found alternative grounds to invalidate the patent, holding that the asserted claims “invalid for inherent anticipation by the ’196 patent under § 102(a).”
Judge Posner had rejected this argument because Apotex “did not prove by clear and convincing evidence that it was impossible to make pure PHC anhydrate in the United States before the critical date.” The appellate panel, however, found that Judge Posner erred by requiring Apotex to meet this standard of proof, “which is too exacting.”
Apotex did not need to prove that it was impossible to make PHC anhydrate in the United States that contained no PHC hemihydrate, but merely that “the disclosure [of the prior art] is sufficient to show that the natural result flowing from the operation as taught [in the prior art] would result in” the claimed product.
Links:
- File Attachment: SKB Apotex en banc order (49 KB)
- File Attachment: SKB Apotex Panel Decision (230 KB)
- SKB Apotex Original Panel Decision
- File Attachment: SKB Apotex District Court.pdf (2443 KB)
- Discussion of the original panel opinion: Part I, Part II, Part III, and part IV.
- Howard Bashman provides an excellent commentary on the case via one of his readers.
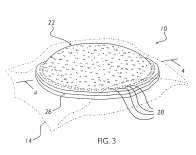
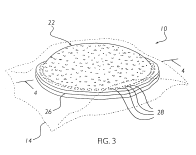 In what may be rival as the swiftest justice in the history of the Court of Appeals for the Federal Circuit. On April 8, only two days after hearing oral arguments, the CAFC rejected Smuckers appeal. The case, in re Kretchman, was widely covered media and involved Smuckers attempt to obtain a broad patent on its Uncrustables(TM).
In what may be rival as the swiftest justice in the history of the Court of Appeals for the Federal Circuit. On April 8, only two days after hearing oral arguments, the CAFC rejected Smuckers appeal. The case, in re Kretchman, was widely covered media and involved Smuckers attempt to obtain a broad patent on its Uncrustables(TM). 
 IP Law Bulletin has a nice
IP Law Bulletin has a nice  Several months ago, I implemented an e-mail newsletter to provide updates to the Patently-O patent law blog. Like the blog, the newsletter is free. List members receive a daily e-mail (usually between 1 and 4 a.m. EST) that contains any new articles from the previous 24 hours. By all accounts, the newsletter is a smashing success — one reader recently wrote in that she loves reading Patently-O every morning. “I don’t drink coffee,” she writes, “so you are my caffeine kick.”
Several months ago, I implemented an e-mail newsletter to provide updates to the Patently-O patent law blog. Like the blog, the newsletter is free. List members receive a daily e-mail (usually between 1 and 4 a.m. EST) that contains any new articles from the previous 24 hours. By all accounts, the newsletter is a smashing success — one reader recently wrote in that she loves reading Patently-O every morning. “I don’t drink coffee,” she writes, “so you are my caffeine kick.” If you have not yet signed up, why not give it a try today? If you decide that you do not like getting the e-mail, you can unsubscribe quite easily. Each daily e-mail includes an “unsubscribe” link. All that you have to do is click on the link and your subscription will be canceled.
If you have not yet signed up, why not give it a try today? If you decide that you do not like getting the e-mail, you can unsubscribe quite easily. Each daily e-mail includes an “unsubscribe” link. All that you have to do is click on the link and your subscription will be canceled. 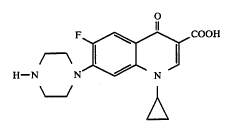 In re Ciprofloxacin Hydrochloride Antitrust Litigation (E.D.N.Y. March 31, 2005)
In re Ciprofloxacin Hydrochloride Antitrust Litigation (E.D.N.Y. March 31, 2005) On April 6 at 2:00 pm, Judges Clevenger, Gajarsa & Prost will hear in re Kretchman (Case No. 04–1448) that involves peanut butter and jelly sandwich technology. Smuckers has already received one patent on its highly profitable “
On April 6 at 2:00 pm, Judges Clevenger, Gajarsa & Prost will hear in re Kretchman (Case No. 04–1448) that involves peanut butter and jelly sandwich technology. Smuckers has already received one patent on its highly profitable “ Teva Pharmaceuticals v. Pfizer (Fed. Cir. 2005) (On petition for rehearing).
Teva Pharmaceuticals v. Pfizer (Fed. Cir. 2005) (On petition for rehearing).  The first Patently-O Patent Prosecution TipCast has just been released. The TipCast series is an audio lecture series that is intended to provide helpful hints and information to patent attorneys and agents. TipCast No. 1 provides a tip relating to revival of abandoned applications.
The first Patently-O Patent Prosecution TipCast has just been released. The TipCast series is an audio lecture series that is intended to provide helpful hints and information to patent attorneys and agents. TipCast No. 1 provides a tip relating to revival of abandoned applications. 
 federal courts that have offended IPO members by denying or refusing to enforce patents. Money saved by cutting these unnecessary government jobs can be used to create a federal program to subsidize businesses known at “patent trolls,” in order to increase patent licensing income and strengthen the U.S. economy. (IPO April 1 Special Report).
federal courts that have offended IPO members by denying or refusing to enforce patents. Money saved by cutting these unnecessary government jobs can be used to create a federal program to subsidize businesses known at “patent trolls,” in order to increase patent licensing income and strengthen the U.S. economy. (IPO April 1 Special Report). In re Fujimura (Fed. Cir. 2005) (NONPRECEDENTIAL).
In re Fujimura (Fed. Cir. 2005) (NONPRECEDENTIAL).  Outlast Technologies v. Frisby Technologies (Fed. Cir. 2005)(Nonprecedential).
Outlast Technologies v. Frisby Technologies (Fed. Cir. 2005)(Nonprecedential). 
 U.S. Patent No.
U.S. Patent No.