Tag Archives: anticipation
Eighth Circuit Decides Patent Case — Adopts Federal Circuit Precedent
En Banc Federal Circuit Panel Changes The Law of Claim Construction
CAFC appears to give implicit deference in claim construction.
Both Parties Lose Patent in Interference Proceeding
Prima Tek II v. Polypap
“Optional” disclosure in prior art reference overcomes negative claim limitation.
SmithKline Beecham v. Apotex: A Conclusion?
Eli Lilly wins patent ruling on Zyprexa
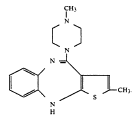
In a much anticipated decision, Judge Richard Young of the Southern District of Indiana has ruled that Eli Lilly’s patent covering the drug Zyprexa is valid, enforceable and infringed. The patent is not scheduled to expire until 2011.
The well-written 224 page opinion reads like a biography of Patent No. 5,229,382, the Zyprexa patent. In particular, the court details the prosecution history of the patent and provides a full analysis of the prior art references that the generic manufacturers cited against the patent. In the end, court determined that the generics could not prove invalidity or unenforceability:
Defendants have failed to prove by clear and convincing evidence that claims 1, 2, 3, 7, 8, and 15 of the ’382 patent are invalid as anticipated under 35 U.S.C. § 102, as obvious under 35 U.S.C. § 103, under the double patenting doctrine, or as barred by prior public use under 35 U.S.C. § 102. Defendants have further failed to prove by clear and convincing evidence that the ’382 patent is unenforceable for inequitable conduct.
The defendant generic manufacturers had stipulated that if the ’382 patent is valid and enforceable, then their actions constitute infringement. Thus, the court found that their ANDA submissions constituted an act of infringement.
SmithKline Beecham v. Apotex part VI
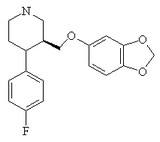
Rather than offering any opinion, the CAFC en banc decision simply vacated the court’s decision. At the same time that the en banc order was released, the three-member appellate panel released their rewritten opinion that avoided the experimental use issue entirely by finding an alternative grounds for finding the patent invalid. Specifically, the court (J. Rader) found that the patent was “inherently anticipated.”
What makes the case even more odd is that, Judge Newman’s dissent from the order declining to hear the case en banc was essentially a written as a dissent from the new Rader decision. Judge Newman criticized the new opinion’s expansion of the doctrine of inherent anticipation, arguing that the “breadth of the panel’s theory of inherent anticipation contravenes long-established precedent.” According to the dissent, if a compound’s existence “is not reasonably known to persons of skill in the field, its later discovery cannot be retrospectively ‘inherently anticipated.’”
According to the new decision, however, inherent anticipation does not require a person of ordinary skill in the art to recognize the inherent disclosure in the prior art at the time the prior art is created. Thus, it appears that a defendant, in order to invalidate a patent, must simply show that, more likely than not, a natural result flowing from an operation taught in the prior art would result in the claimed product.
In this case, the recreated panel found that the prior art was sufficient because it disclosed the manufacture of PHC anhydrate that naturally results in the production of the claimed compound (PHC hemihydrate). However, the court stated that a mere possibility of inherency is not sufficient for anticipation. (Citing Schering Corp.).
In concurrence, Judge Gajarsa found that the manufacture of PHC hemihydrate could be “a natural physical process” occurring “under normal climactic conditions and with no human intervention,” and thus found that it was an unpatentable process of nature.
In short, patent claims drawn broadly enough to encompass products that spread, appear, and “reproduce” through natural processes cover subject matter unpatentable under Section 101–and are therefore invalid.
Gajarsa’s concurrence is a foreshadow to the next few years where patentable subject matter will become a controversial area once again.
Links:
- File Attachment: SKB Apotex en banc order (49 KB)
- File Attachment: SKB Apotex Panel Decision (230 KB)
- SKB Apotex Original Panel Decision (From Georgetown Law Center)
- File Attachment: SKB Apotex District Court.pdf (2443 KB)
- Discussion of the original panel opinion: Part I, Part II, Part III, and part IV.
- Discussion of the en banc order and new appellate opinion: Part V, Part VI
- Howard Bashman provides an excellent commentary on the case via one of his readers.
- Information on SKB’s pay-out in the class action antitrust suit. (Link).
Federal Circuit Makes En Banc Decision in Paxil Case: Patent Inherently Anticipated
SmithKline Beecham Corp. v. Apotex Corp. (Fed. Cir. 2005) (en banc order)
Paxil(R) is a blockbuster drug sold by SmithKline Beecham (SKB). SKB’s patent covering the drug was initially invalidated in an opinion by 7th Circuit Judge Richard A. Posner who sat by designation as a district court judge. On appeal, a Federal Circuit panel (J. Rader) rejected almost all of Judge Posner’s reasoning, but affirmed the invalidation decision on other grounds.
Now, the CAFC issued an en banc decision to vacate the panel’s original decision with regard to the issue of experimental use, and the panel (J. Rader) has issued its new decision.
This case involves involved PHC anhydrates (without water) and PHC hemihydrates (some water). In the 70’s PHC anhydrates were invented in the UK. In 1984, SKB invented PHC hemihydrates and eventually patented a PHC hemihydrate compound. (U.S. Patent No. 4,721,723). In 1998, Apotex filed an ANDA to market a prior art PHC anhydrate. The question of experimental use arises because SKB performed clinical trials that occurred more than one year prior to filing of the patent application. Normally, a public use of a patented invention more than a year before filing invalidates the patent under 35 U.S.C. §102(b). However SKB has argued that the clinical trials were a form of experimental use.
In its new decision, the CAFC majority simply avoided the issue of experimental public use in totality. Rather, they found alternative grounds to invalidate the patent, holding that the asserted claims “invalid for inherent anticipation by the ’196 patent under § 102(a).”
Judge Posner had rejected this argument because Apotex “did not prove by clear and convincing evidence that it was impossible to make pure PHC anhydrate in the United States before the critical date.” The appellate panel, however, found that Judge Posner erred by requiring Apotex to meet this standard of proof, “which is too exacting.”
Apotex did not need to prove that it was impossible to make PHC anhydrate in the United States that contained no PHC hemihydrate, but merely that “the disclosure [of the prior art] is sufficient to show that the natural result flowing from the operation as taught [in the prior art] would result in” the claimed product.
Links:
- File Attachment: SKB Apotex en banc order (49 KB)
- File Attachment: SKB Apotex Panel Decision (230 KB)
- SKB Apotex Original Panel Decision
- File Attachment: SKB Apotex District Court.pdf (2443 KB)
- Discussion of the original panel opinion: Part I, Part II, Part III, and part IV.
- Howard Bashman provides an excellent commentary on the case via one of his readers.