Tag Archives: Enablement
Biotech: Written Description Does Not Require Recitation Of A Known Structure
DJ Counterclaims Allow Adjudication of Invalidity Counterclaims Even After Noninfringement Decision
Should the Patent Office Kill Late Claiming?: Proposed Continuation Change
Federal Circuit Further Limits Doctrine of Equivalents under “Specific Exclusion” Principle
CAFC Explains Materiality Standard for Inequitable Conduct
Supreme Court: LabCorp Briefing Round I [UPDATED]
Under 102(g), Conception of Prior Art Requires Appreciation of Invention
CAFC reverses jury finding of invalidity and noninfringement.
USPTO Releases New Business Method Guidelines: Requires “physical transformation” or “concrete and tangible result”
Single Embodiment Of Claim Element Fails To Describe Element Generically
Including Claims in Provisional Patent Applications?
CAFC: Genetic Tags Unpatentable Unless Function of Underlying Gene is Described
RIM, with the support of Canada and Intel, Ask Court for Another Review of BlackBerry Patent Case
Incomplete Written Analysis Results in Reversal of Summary Judgment
Both Parties Lose Patent in Interference Proceeding
PTO Board: Disclosure of Sequence Enables at least 5% of Natural Variance.
Indecipherable Patents
March 2005 Report on New Academic Research
Each month I post a note discussing new research from the academic side of patent law. This March edition includes three great articles that relate to either legislative changes (prior use statute, post grant opposition) or upcoming court decisions (Phillips claim construction case). Feel free to e-mail suggestions for April’s edition.
- Tom Fairhall and Paul Churilla, Prior Use of Trade Secrets and the Intersection with Patent Law, 14 Fed. Cir. Bar J. 455. Fairhall and Churilla present a timely article on prior use rights that notes how the current prior use statute (35 USC 273) does not provide the scope necessary to truly protect a prior user’s expectations or investments. The article is quite timely because FTC proposals to expand the statutory prior use rights is gaining some teeth in the patent community and on the Hill.
- Mark Lemley, The Changing Meaning of Patent Claim Terms, available at SSRN. Professor Lemley’s article is also timely — he discusses at what particular point in time should the meaning of claim terms be fixed. This is important because the ordinary meaning of terms in the English language change over time. The major temporal candidates are (i) at the time of the invention (currently used for novelty and nonobviousness); (ii) at the time the application was filed (currently used for enablement or written description); (iii) a the time the patent issues (currently used for means-plus-function claim construction); and (iv) at the time of the infringement (at least some times used to determine infringement). Lemley agrees that each candidate has a sound legal foundation. However, he argues that the best approach moving forward is to fix the meaning at the time the patent application is first filed.
- Jay Kesan and Andres Gallo, Why ‘Bad’ Patents Survive in the Market, available at SSRN. The professors provide a game theory model to show how would-be invalid patents can survive and be valuable in the marketplace. Essentially, the transaction costs make it difficult to mount an effective challenge to improperly granted patents. Their work will serve as the theoretical basis any proposed post-grant opposition procedures. Specifically, the authors “conclude that a low-cost, post-grant opposition process based primarily on written submissions within a limited estoppel effect . . . will serve as an effective instrument for improving the quality of patents that are issued and enforced.”
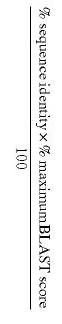 Ex parte Bandman, No. 2004-2319, (BPAI 2005)
Ex parte Bandman, No. 2004-2319, (BPAI 2005) 