SmithKline Beecham v. Apotex (Fed. Cir. 2006, 04–1522).
SmithKline’s U.S. Patent No. 6,113,944 is directed to paroxetine (Paxil) made through an allegedly novel process (product-by-process). In 1998, Apotex filed its ANDA and paragraph IV certification asserting that the patent was invalid — thus provoking action from SmithKline.
On summary judgment, the district court found the patent invalid as anticipated by prior knowledge of paroxetine. Specifically, the lower court determined that the claimed process steps were not limiting. Thus, any prior disclosure of the end-product was anticipating.
Product-by-Process: On appeal, the CAFC affirmed, finding that product-by-process claims cannot recapture the end-product from the public domain.
[O]nce a product is fully disclosed in the art, future claims to that same product are precluded, even if that product is claimed as made by a new process.
This rule of law follows the MPEP, which states that:
[Even] though product-by-process claims are limited by and defined by the process, determination of patentability is based on the product itself. The patentability of a product does not depend on its method of production. If the product in the product-by-process claim is the same as or obvious from a product of the prior art, the claim is unpatentable even though the prior product was made by a different process.
MPEP § 2113 (8th ed., Rev. 2, May 2004) (quoting In re Thorpe, 777 F.2d at 698).
Differences from Prior-Art Product: In a footnote, SmithKline’s attorneys noted that the product made through the claimed process is actually different (lacked a pink hue and did not contain spherical granules). The district court ignored these differences because they were “not required by the patent claims or specification.”
However, the appellate panel the differences at least interesting — “If those product-by-process claims produced a different product than that disclosed by the [prior art], there would be an argument that the [prior art] disclosure did not anticipate.” In the end, the panel refused to consider the issue because it was only raised in a footnote:
This footnote is the only statement that even approaches a substantive argument on novelty in the entire Argument section of SmithKline’s opening brief. . . . We find that these mere statements of disagreement with the district court as to the existence of factual disputes do not amount to a developed argument. . . . Further, arguments raised in footnotes are not preserved.
Invalidity affirmed.
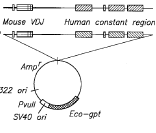 MedImmune v. Genentech* (Supreme Court 2006).
MedImmune v. Genentech* (Supreme Court 2006).  Lawman Armor v. Winner Int’l (Fed. Cir. 2006,
Lawman Armor v. Winner Int’l (Fed. Cir. 2006, 

 Math You Can’t Use
Math You Can’t Use
 Nautilus v. ICON (Fed. Cir. 2006,
Nautilus v. ICON (Fed. Cir. 2006,  In dissent, Judge Newman essentially argued that the practical nature of claim construction appeals should allow all claim construction issues to be reviewed at one time.
In dissent, Judge Newman essentially argued that the practical nature of claim construction appeals should allow all claim construction issues to be reviewed at one time.  Golden Blount v. Robert H. Peterson Co. (Fed. Cir. 2006,
Golden Blount v. Robert H. Peterson Co. (Fed. Cir. 2006,