by Dennis Crouch
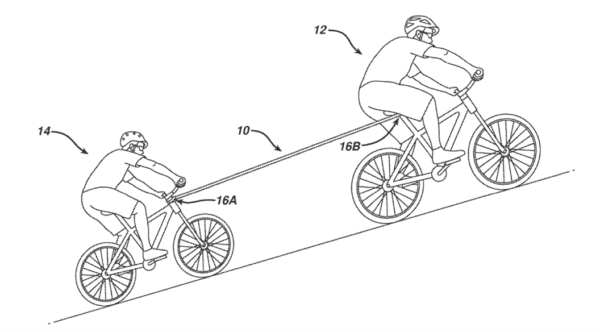
In Roku, Inc. v. ITC, the Federal Circuit has affirmed determinations by the International Trade Commission (“ITC”) favoring the patent holder Universal Electronics, Inc. (“Universal”). The most interesting part of the case for me is the assignment issue - whether the patents had been properly assigned at the appropriate time. This can become in cases like this because Universal has created a large patent portfolio that all claim back to original priority documents from more than a decade ago. While most of patents are attributable to both joint-inventors, some are only attributable to one or the other. Here, though the Federal Circuit supported the simple approach of a "hereby assigns" transfer of rights that includes future continuations. The decision is lacking though because the court does not ground its decision in any particular contract or property tradition.
To continue reading, become a Patently-O member. Already a member? Simply log in to access the full post.

 Congratulations to Sharon Israel on her new leadership role at the USPTO. I was happy to learn that Director Vidal has appointed Sharon Israel as its new Chief Policy Officer and Director for International Affairs. Ms. Israel has been a leader of the patent bar for many yeas and will bring tremendous expertise to this important role overseeing the USPTO's policy and international programs. For the past few years, she has been a partner at Shook Hardy focusing on patent litigation -- primarily on the defense side.
Congratulations to Sharon Israel on her new leadership role at the USPTO. I was happy to learn that Director Vidal has appointed Sharon Israel as its new Chief Policy Officer and Director for International Affairs. Ms. Israel has been a leader of the patent bar for many yeas and will bring tremendous expertise to this important role overseeing the USPTO's policy and international programs. For the past few years, she has been a partner at Shook Hardy focusing on patent litigation -- primarily on the defense side.
 by Dennis Crouch
by Dennis Crouch